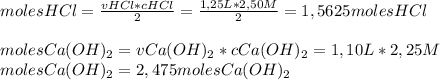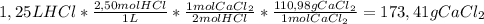Chemistry, 24.07.2019 08:00, freshysans4
How many grams of calcium chloride would form if 1.25 liters of a 2.50 molar hydrochloric acid (hcl) solution reacts with 1.10 liters of a 2.25 molar calcium hydroxide ca(oh)2 solution? (2 points) 2hcl (aq) + ca(oh)2 (aq) yields cacl2 (aq) + h2o (l)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
Do you know the correct answer?
How many grams of calcium chloride would form if 1.25 liters of a 2.50 molar hydrochloric acid (hcl)...
Questions in other subjects:




Mathematics, 19.01.2020 19:31

Mathematics, 19.01.2020 19:31

Biology, 19.01.2020 19:31



Arts, 19.01.2020 19:31