
Chemistry, 24.07.2019 12:30, jayowens20
For the reaction x2 + y + z → xy + xz, it is found that doubling the concentration of x2 doubles the reaction rate, tripling the concentration of y triples the rate, and doubling the concentration of z has no effect. what is the rate law for this reaction?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
Do you know the correct answer?
For the reaction x2 + y + z → xy + xz, it is found that doubling the concentration of x2 doubles the...
Questions in other subjects:



Mathematics, 02.03.2021 04:40

Mathematics, 02.03.2021 04:40

Mathematics, 02.03.2021 04:40




Biology, 02.03.2021 04:40

Chemistry, 02.03.2021 04:40


![\boxed{rate=k\left[ {{{\text{X}}_2}}\right]\left[ {\text{Y}} \right]}](/tpl/images/0127/2448/0a486.png) .
.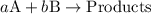
![{\text{rate}}=k{\left[{\text{A}}\right]^a}{\left[{\text{B}}\right]^b}](/tpl/images/0127/2448/dedd1.png) ...... (1)
...... (1)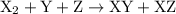
![{\text{rate}}=k{\left[{{{\text{X}}_2}}\right]^a}{\left[{\text{Y}}\right]^b}{\left[ {\text{Z}} \right]^c}](/tpl/images/0127/2448/0b279.png) …… (2)
…… (2)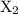 , Y, and Z respectively.
, Y, and Z respectively.![\begin{aligned}{\text{rate}}&=k{\left[{{{\text{X}}_2}}\right]^a}{\left[{\text{Y}}\right]^b}{\left[ {\text{Z}}\right]^c}\\&=k{\left[{{{\text{X}}_2}}\right]^1}{\left[ {\text{Y}}\right]^1}{\left[ {\text{Z}}\right]^0}\\&=k\left[{{{\text{X}}_2}}\right]\left[ {\text{Y}}\right]\\\end{aligned}](/tpl/images/0127/2448/fee78.png)




