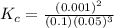Chemistry, 24.07.2019 21:30, knownperson233
In the reaction n2 + 3h2 ⇌ 2nh3, an experiment finds equilibrium concentrations of [n2] = 0.1 m, [h2] = 0.05 m, and [nh3] = 0.001 m. what is the equilibrium constant kc for this reaction? a. 0.08 b. 0.20 c. 5.0 d. 12.5

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 22.06.2019 21:30, Lindsay882
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
Do you know the correct answer?
In the reaction n2 + 3h2 ⇌ 2nh3, an experiment finds equilibrium concentrations of [n2] = 0.1 m, [h2...
Questions in other subjects:











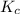 = 0.08
= 0.08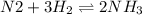
![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0128/7284/c3aa0.png)