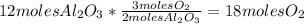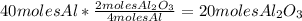Chemistry, 25.07.2019 01:00, ethanhose05
Balance this reaction: al(s) + o2(g) → al2o3(s) how many moles of oxygen will be needed to react with aluminum metal to produce 12.0 moles of aluminum oxide? 6. using the reaction listed in question 5 (above), how many moles of aluminum oxide will be produced by 40.0 moles of aluminum reacting completely with a boat load (that is a lot) of oxygen?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3
Do you know the correct answer?
Balance this reaction: al(s) + o2(g) → al2o3(s) how many moles of oxygen will be needed to react w...
Questions in other subjects:








Mathematics, 02.07.2020 20:01