
Chemistry, 25.07.2019 03:00, ewymer3901
Some antacid tablets contain aluminum hydroxide. the aluminum hydroxide reacts with stomach acid according to the equation: ai(oh)3 +3hciaici, +3h2o. determine the moles of acid neutralized if a tablet contains 0.200 mol of al(oh)3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 07:30, lifeislove3251
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1

Chemistry, 23.06.2019 13:20, mia7791
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas. how many grams of aluminum sulfate would be formed if 250 g h 2 so 4 completely reacted with aluminum? 2al( s ) + 3h 2 so 4 ( aq ) ? al 2 (so 4 ) 3 ( aq ) + 3h 2 ( g )
Answers: 1

Chemistry, 23.06.2019 16:30, sandygarcia65
Which of the following is a way carbon enters the atmosphere volcanic activity photosynthesis deposition of settlement burial of biomass hep hurrryy
Answers: 1
Do you know the correct answer?
Some antacid tablets contain aluminum hydroxide. the aluminum hydroxide reacts with stomach acid acc...
Questions in other subjects:


Biology, 10.11.2020 23:10

History, 10.11.2020 23:10




Mathematics, 10.11.2020 23:10

History, 10.11.2020 23:10


Mathematics, 10.11.2020 23:10


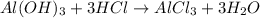
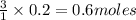 of hydrochloric acid.
of hydrochloric acid.



