Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3



Chemistry, 23.06.2019 06:30, morganzahn16
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
Do you know the correct answer?
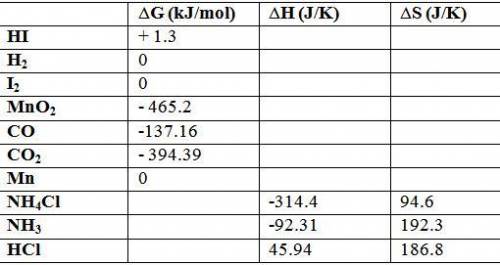
Calculate δg o for each reaction using δg of values: (a) h2(g) + i2(s) → 2hi(g) kj (b) mno2(s) + 2co...
Questions in other subjects:

History, 17.10.2019 06:10

History, 17.10.2019 06:10




Mathematics, 17.10.2019 06:10



History, 17.10.2019 06:10

Mathematics, 17.10.2019 06:10