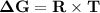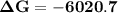Chemistry, 25.07.2019 07:00, zetrenne73
Consider the reaction 2no2(g) → n2o4(g) and δg∘(no2(g)) = 51.84 kj/mol, δg∘(n2o4(g)) = 98.28 kj/mol. calculate δg at 298 k if the partial pressures of no2 and n2o4 are 0.38atm and 1.64atm , respectively.

Answers: 1
Similar questions

Chemistry, 23.06.2019 07:30, jonquil201
Answers: 1

Chemistry, 23.06.2019 17:00, mikemofun9079
Answers: 1


Chemistry, 03.11.2019 20:31, cragajjar2
Answers: 2
Do you know the correct answer?
Consider the reaction 2no2(g) → n2o4(g) and δg∘(no2(g)) = 51.84 kj/mol, δg∘(n2o4(g)) = 98.28 kj/mol....
Questions in other subjects:

Mathematics, 06.01.2021 06:00

Biology, 06.01.2021 06:00

Mathematics, 06.01.2021 06:00





Social Studies, 06.01.2021 06:00


Mathematics, 06.01.2021 06:00