Chemistry, 25.07.2019 08:30, itcelmairani
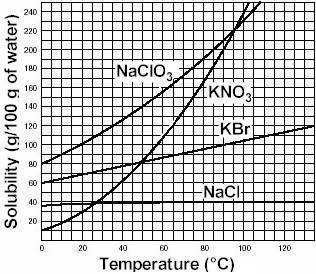
Asolution is a homogeneous mixture of one or more solutes dissolved in a solvent. a specific volume of solvent is only able to dissolve a limited amount of solute. as long as the solvent is able to dissolve more solute, the solution remains unsaturated. when the solvent can no longer dissolve additional solute, the solution is saturated. at this point, any additional solute will fall to the bottom of the container. the amount of solute that can be dissolved in a given amount of solvent at a specific temperature and pressure is defined as the solubility of the solute. consider the solubility curves of several salts in water. based on the information here, if 220 grams of the salt kbr are added to 100 ml of water at 100oc, we would label that solution as a) insoluble b) saturated c) supersaturated d) unsaturated

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
Do you know the correct answer?
Asolution is a homogeneous mixture of one or more solutes dissolved in a solvent. a specific volume...
Questions in other subjects:


Mathematics, 22.09.2021 14:00

Biology, 22.09.2021 14:00

Physics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00


Mathematics, 22.09.2021 14:00