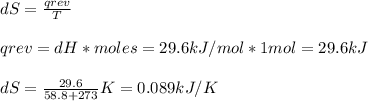Chemistry, 25.07.2019 11:00, kornut7316
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δhvap = 29.6 kj/mol. (a) when br2(l) boils at its normal boiling point, does its entropy increase or decrease? decrease (δs is negative) increase (δs is positive) (b) calculate the value of δs when 1.00 mol of br2(l) is vaporized at 58.8°c.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2
Do you know the correct answer?
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δh...
Questions in other subjects:


English, 09.06.2021 18:50





Chemistry, 09.06.2021 18:50



Mathematics, 09.06.2021 18:50