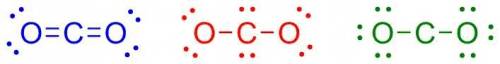Carbon has four valence electrons, and oxygen has six valence electrons. if carbon and oxygen bond covalently, which of the following is the correct lewis dot (electron dot) structure for carbon dioxide? carbon has four valence electrons, and oxygen has six valence electrons. if carbon and oxygen bond covalently, which of the following is the correct lewis dot (electron dot) structure for carbon dioxide? a lewis dot structure with the following configuration: one oxygen atom with four valence electrons, a double bond to a carbon atom, followed by a double bond to a second oxygen atom which has four valence electrons. a lewis dot structure with the following configuration: one oxygen atom with four valence electrons, a single bond to a carbon atom with four valence electrons, followed by a single bond to a second oxygen atom which has four valence electrons. a lewis dot structure with the following configuration: one oxygen atom with six valence electrons, a single bond to a carbon atom, followed by a single bond to a second oxygen atom which has six valence electrons.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, lifeofabe214
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1
Do you know the correct answer?
Carbon has four valence electrons, and oxygen has six valence electrons. if carbon and oxygen bond c...
Questions in other subjects:










English, 21.06.2019 15:00