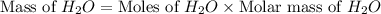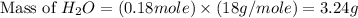Gaseous methane ch4 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 1.44 g of methane is mixed with 9.5 g of oxygen. calculate the maximum mass of water that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:30, jaylenmiller437
The diagram shows the relationship between scientific disciplines. the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a. physics b. biology c. chemistry d. metallurgy
Answers: 2
Do you know the correct answer?
Gaseous methane ch4 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gase...
Questions in other subjects:




Health, 04.08.2019 05:00

Physics, 04.08.2019 05:00




Spanish, 04.08.2019 05:00


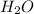 produced will be, 3.24 grams
produced will be, 3.24 grams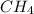 = 1.44 g
= 1.44 g
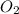 = 9.5 g
= 9.5 g
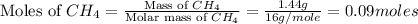
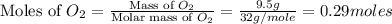

 moles of
moles of