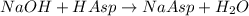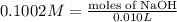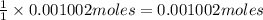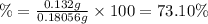Chemistry, 26.07.2019 03:30, codyshs160
Astudent found that the titration had taken 10.00 ml of 0.1002 m naoh to titration 0.132 g of aspirin, a monoprotic acid. calculate the percent purity of aspirin (c9h8o4, molar mass = 180.2 g/mol) sample.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, markipler01
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2
Do you know the correct answer?
Astudent found that the titration had taken 10.00 ml of 0.1002 m naoh to titration 0.132 g of aspiri...
Questions in other subjects:


History, 29.09.2019 14:50







Business, 29.09.2019 14:50

Biology, 29.09.2019 14:50