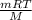Many gases are shipped in high-pressure containers. consider a steel tank whose volume is 55.0 gallons that contains o2 gas at a pressure of 16,500 kpa at 23 °c. (a) what mass of o2 does the tank contain? (b) what volume would the gas occupy at stp? (c) at what temperature would the pressure in the tank equal 150.0 atm? (d) what would be the pressure of the gas, in kpa, if it were transferred to a container at 24 °c whose volume is 55.0 l?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
Do you know the correct answer?
Many gases are shipped in high-pressure containers. consider a steel tank whose volume is 55.0 gallo...
Questions in other subjects:


Mathematics, 22.06.2019 12:00