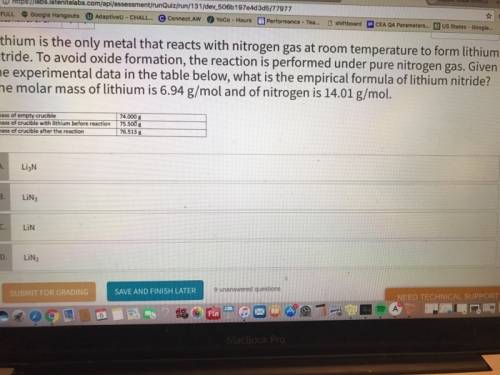
Lithium is the only metal that reacts with nitrogen gas at room temperature to form lithium nitride. to avoid oxide formation, the reaction is performed under pure nitrogen gas. given the experimental data in the table below, what is the empirical formula of lithium nitride? the molar mass of lithium is 6.94 g/mol and of nitrogen is 14.01 g/mol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1


Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
Do you know the correct answer?
Lithium is the only metal that reacts with nitrogen gas at room temperature to form lithium nitride....
Questions in other subjects:



History, 03.12.2021 16:50



History, 03.12.2021 16:50

English, 03.12.2021 16:50

Physics, 03.12.2021 16:50

Social Studies, 03.12.2021 16:50