
Chemistry, 26.07.2019 20:30, wyattgrubb00
Which equivalence factor set should you use to convert from 4.23 x 1012 atoms of ca to grams of ca? a) (1 mol ca/6.02 x 1023 atoms ca)(40.08 g ca/1 mol ca) b) (1 mol ca/4.23 x 1012 atoms ca)(40.08 g ca/1 mol ca) c) (4.23 x 1012 atoms ca/1 mol ca)(1 mol ca/40.08 g ca) d) 4.23 x 1012 atoms ca/6.02 x 1023 atoms ca)(40.08 g ca)

Answers: 1
Similar questions


Chemistry, 01.09.2019 02:30, chriscol4082
Answers: 1


Chemistry, 11.10.2019 07:30, zoel222
Answers: 3
Do you know the correct answer?
Which equivalence factor set should you use to convert from 4.23 x 1012 atoms of ca to grams of ca?...
Questions in other subjects:

Mathematics, 20.04.2020 20:06





History, 20.04.2020 20:06

History, 20.04.2020 20:06

Mathematics, 20.04.2020 20:06


Mathematics, 20.04.2020 20:06


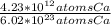 * 40.08 g Ca
* 40.08 g Ca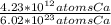 * 40.08 g Ca
* 40.08 g Ca



