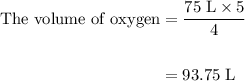Acetylene gas (c2h2) reacts with oxygen gas (o2) to produce carbon dioxide (co2) and water vapor (h2o). how many liters of c2h2 are required to produce 75.0 l of co2? l what volume of h2o is produced? l what volume of o2 is required? l when making the calculations, did you need to find the number of moles?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1


Chemistry, 22.06.2019 04:00, kichensides
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1
Do you know the correct answer?
Acetylene gas (c2h2) reacts with oxygen gas (o2) to produce carbon dioxide (co2) and water vapor (h2...
Questions in other subjects:


English, 04.03.2021 02:30


Mathematics, 04.03.2021 02:30

Computers and Technology, 04.03.2021 02:30


Mathematics, 04.03.2021 02:30

Mathematics, 04.03.2021 02:30

Mathematics, 04.03.2021 02:30


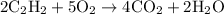
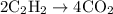
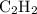 = 4 L of
= 4 L of 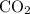
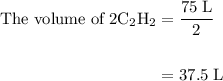
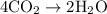
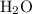
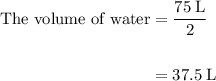
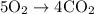
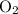 = 4 L of
= 4 L of