
Chemistry, 27.07.2019 01:00, epicsushiman1
How many moles of carbon dioxide (co2) are produced when reacting 6.00 moles of butane (c4h10) in excess oxygen (o2)?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
Do you know the correct answer?
How many moles of carbon dioxide (co2) are produced when reacting 6.00 moles of butane (c4h10) in ex...
Questions in other subjects:

Health, 08.07.2019 03:50


Mathematics, 08.07.2019 03:50

Business, 08.07.2019 03:50

Mathematics, 08.07.2019 03:50






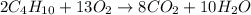
 of carbon dioxide.
of carbon dioxide.



