
Chemistry, 27.07.2019 05:30, timothymoles
The theoretical yield for a reaction is 55.9 g licl. the actual yield is 24.6 g licl. what is the percent yield of the reaction? question options: 227% 44% 25% 56%

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 01:00, dawnparker71
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
Do you know the correct answer?
The theoretical yield for a reaction is 55.9 g licl. the actual yield is 24.6 g licl. what is the pe...
Questions in other subjects:





Mathematics, 23.06.2019 08:40

Mathematics, 23.06.2019 08:40




Mathematics, 23.06.2019 08:40


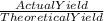 × 100
× 100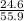 × 100
× 100



