Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, angeljohnson2081
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2


Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
Do you know the correct answer?
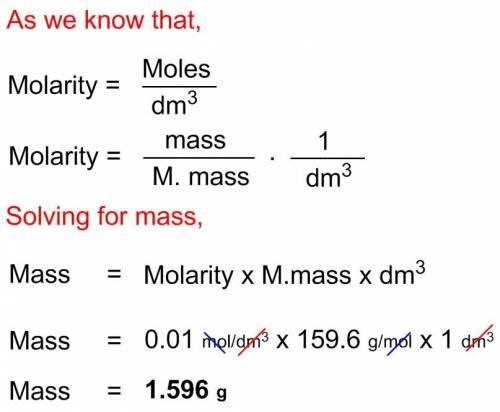
Asolution of cuso4 is labelled 0.01 m. how much cuso4 in grams, must be used to make 1 liter of solu...
Questions in other subjects:

Mathematics, 29.09.2019 21:30

History, 29.09.2019 21:30

Health, 29.09.2019 21:30

English, 29.09.2019 21:30

Social Studies, 29.09.2019 21:30

Mathematics, 29.09.2019 21:30

Mathematics, 29.09.2019 21:30

Mathematics, 29.09.2019 21:30