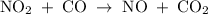The reaction between no2 and co to produce no and co2 is thought to occur in two steps: step 1: no2 + no2 no + no3 step 2: no3 + co no2 + co2 the experimental rate law is rate = k[no2]2. (a) write the equation for the overall reaction. do not include phase abbreviations. (b) identify the intermediate(s). no3 no2 co no co2

Answers: 1
Similar questions

Mathematics, 23.07.2019 04:20, hastlingc
Answers: 2

Mathematics, 21.09.2019 22:30, Sumitco9578
Answers: 2

Chemistry, 23.09.2019 02:50, alma79
Answers: 2

Mathematics, 26.10.2019 22:43, J3ak06
Answers: 1
Do you know the correct answer?
The reaction between no2 and co to produce no and co2 is thought to occur in two steps: step 1: no...
Questions in other subjects:

English, 31.08.2019 01:30

History, 31.08.2019 01:30

History, 31.08.2019 01:30


Physics, 31.08.2019 01:30


Chemistry, 31.08.2019 01:30

Social Studies, 31.08.2019 01:30

Chemistry, 31.08.2019 01:30

English, 31.08.2019 01:30


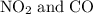 to produce NO and
to produce NO and 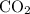 occurs in the given two steps.
occurs in the given two steps.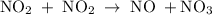

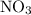 because it act as an intermediate and is utilized in the next step.
because it act as an intermediate and is utilized in the next step.