

Answers: 1
Similar questions



Chemistry, 19.11.2019 02:31, christophers349
Answers: 2

Chemistry, 19.11.2019 07:31, bshreve
Answers: 1
Do you know the correct answer?
The nickel(ii) ion is commonly dissolved in solution using nickel(ii) nitrate hexahydrate, ni(no3)2....
Questions in other subjects:


Mathematics, 10.09.2021 06:10


Mathematics, 10.09.2021 06:10




Mathematics, 10.09.2021 06:10



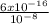 = 6 x 10⁻⁸ M
= 6 x 10⁻⁸ M



