
Chemistry, 27.07.2019 13:00, Clervoyantyvonne
Given the equation representing a reaction at equilibrium: h2s(aq) ch3nh2(aq) hs(aq) ch3nh3 (aq) according to one acid-base theory, the forward reaction is classified as an acid-base reaction because

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Do you know the correct answer?
Given the equation representing a reaction at equilibrium: h2s(aq) ch3nh2(aq) hs(aq) ch3nh3 (aq) ac...
Questions in other subjects:


Chemistry, 28.07.2019 18:00

History, 28.07.2019 18:00


Mathematics, 28.07.2019 18:00

Biology, 28.07.2019 18:00


History, 28.07.2019 18:00



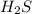 loses proton and
loses proton and 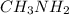 accepts proton
accepts proton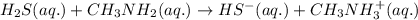
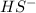 which is a conjugate base.
which is a conjugate base.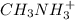 which is a conjugate acid.
which is a conjugate acid.



