Chemistry, 27.07.2019 17:30, nockturnal1993
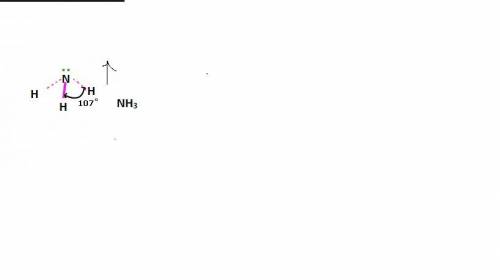
In ammonia, a central nitrogen atom is bonded to three hydrogen atoms. similarly, boron trifluoride has a central boron atom bonded to three fluorine atoms. however, ammonia is pyramidal and boron trifluoride is trigonal planar in shape. which statement justifies this difference in their structure? the lone pair of electrons in boron trifluoride increases the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in boron trifluoride decreases the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in boron trifluoride stabilizes the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in ammonia increases the bond angle between the bonding pairs, giving it a pyramidal structure. the lone pair of electrons on the nitrogen in ammonia decreases the bond angle between the bonding pairs, giving it a pyramidal structure.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1


Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
Do you know the correct answer?
In ammonia, a central nitrogen atom is bonded to three hydrogen atoms. similarly, boron trifluoride...
Questions in other subjects:

Mathematics, 25.06.2019 03:30




Physics, 25.06.2019 03:30

English, 25.06.2019 03:30



History, 25.06.2019 03:30


![:{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0139/5742/08b6a.png)
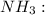
![{\text{Number of electrons}} =\frac{1}{2}[5+3-0+0]=4](/tpl/images/0139/5742/46c6e.png)
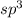 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.
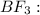
![{\text{Number of electrons}} =\frac{1}{2}[3+3-0+0]=3](/tpl/images/0139/5742/beb45.png)
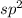 and the electronic geometry of the molecule will be trigonal planar.
and the electronic geometry of the molecule will be trigonal planar.