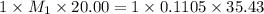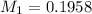You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you will titrate this with a 0.1105 m naoh. you add 20.00 ml of hcooh to the beaker before titrating, and it requires 35.43 ml of naoh to reach the end point. what is the concentration of the hcooh solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, NREYESLDS2806
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 23.06.2019 06:20, cowboo5000pcl655
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 09:00, floressavanna15
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
Do you know the correct answer?
You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you...
Questions in other subjects:


English, 28.03.2020 01:38




History, 28.03.2020 01:38




Mathematics, 28.03.2020 01:38



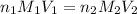
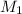 = molarity of
= molarity of 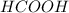 solution = ?
solution = ?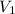 = volume of
= volume of  = molarity of
= molarity of 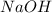 solution = 0.1105 M
solution = 0.1105 M
 = volume of
= volume of 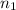 = valency of
= valency of 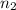 = valency of
= valency of