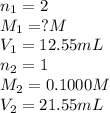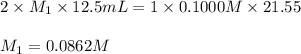Chemistry, 28.07.2019 03:30, dessyrob05
Asolution of malonic acid, h2c3h2o4 , was standardized by titration with 0.1000 m naoh solution. if 21.55 ml of the naoh solution were required to neutralize completely 12.55 ml of the malonic acid solution, what is the molarity of the malonic acid solution?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Do you know the correct answer?
Asolution of malonic acid, h2c3h2o4 , was standardized by titration with 0.1000 m naoh solution. if...
Questions in other subjects:

English, 19.01.2021 18:10

Arts, 19.01.2021 18:10


Mathematics, 19.01.2021 18:10

Social Studies, 19.01.2021 18:10

English, 19.01.2021 18:10

Mathematics, 19.01.2021 18:10


Mathematics, 19.01.2021 18:10


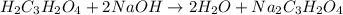
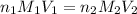
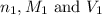 are the n-factor, molarity and volume of malonic acid.
are the n-factor, molarity and volume of malonic acid.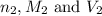 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.