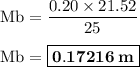Chemistry, 28.07.2019 07:00, carolineepoolee84
A25.00 −ml sample of an unknown hclo4 solution requires titration with 21.52 ml of 0.2000 m naoh to reach the equivalence point. part a what is the concentration of the unknown hclo4 solution? the neutralization reaction is: hclo4(aq)+naoh(aq)→h2o(l)+naclo4(aq )

Answers: 1
Similar questions



Chemistry, 20.10.2019 18:10, getsic
Answers: 3

Chemistry, 05.11.2019 05:31, 4300224102
Answers: 2
Do you know the correct answer?
A25.00 −ml sample of an unknown hclo4 solution requires titration with 21.52 ml of 0.2000 m naoh to...
Questions in other subjects:





Mathematics, 05.05.2020 13:21



Chemistry, 05.05.2020 13:21

Mathematics, 05.05.2020 13:21

Mathematics, 05.05.2020 13:21