Chemistry, 28.07.2019 13:00, naimareiad
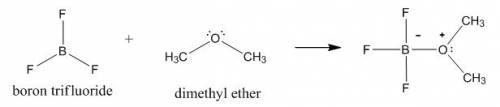
Draw the lewis structure of the 1: 1 adduct that forms in the lewis acid-base reaction between boron trifluoride (bf3) and dimethyl ether ((ch3)2o).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Do you know the correct answer?
Draw the lewis structure of the 1: 1 adduct that forms in the lewis acid-base reaction between boron...
Questions in other subjects:


Mathematics, 11.01.2020 03:31


Mathematics, 11.01.2020 03:31


Mathematics, 11.01.2020 03:31