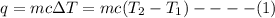Chemistry, 28.07.2019 20:30, unknowntay04
35.5 grams of an unknown substance is heated to 103.0 degrees celsius and then placed into a calorimeter containing 100.0 grams of water at 24.0 degrees celsius. if the final temperature reached in the calorimeter is 29.5 degrees celsius, what is the specific heat of the unknown substance?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
Do you know the correct answer?
35.5 grams of an unknown substance is heated to 103.0 degrees celsius and then placed into a calorim...
Questions in other subjects:


Mathematics, 25.06.2019 12:30



Biology, 25.06.2019 12:30

Chemistry, 25.06.2019 12:30


Social Studies, 25.06.2019 12:30

Mathematics, 25.06.2019 12:30