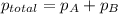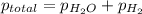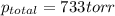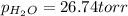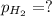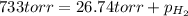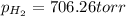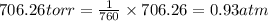Chemistry, 29.07.2019 01:30, dasdsadsafdhgifsdu
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: zn(s) + h2so4(aq) → znso4(aq) + h2(g) in an experiment, 201 ml of wet h2 is collected over water at 27°c and a barometric pressure of 733 torr. the vapor pressure of water at 27°c is 26.74 torr. the partial pressure of hydrogen in this experiment is atm.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
Do you know the correct answer?
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: zn(s) + h2so4(aq) → znso4(aq) + h2(g)...
Questions in other subjects:


Physics, 04.12.2020 18:00

Social Studies, 04.12.2020 18:00



English, 04.12.2020 18:00


Chemistry, 04.12.2020 18:00