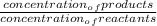Chemistry, 29.07.2019 01:30, hollisjune3047
Yet another reaction has an equilibrium constant kc=4.32×105 at 25 ∘c. it is an exothermic reaction, giving off quite a bit of heat while the reaction proceeds. if the temperature is raised to 200 ∘c , what will happen to the equilibrium constant? the equilibrium constant will yet another reaction has an equilibrium constant at 25 . it is an exothermic reaction, giving off quite a bit of heat while the reaction proceeds. if the temperature is raised to 200 , what will happen to the equilibrium constant?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
Do you know the correct answer?
Yet another reaction has an equilibrium constant kc=4.32×105 at 25 ∘c. it is an exothermic reaction,...
Questions in other subjects:

Chemistry, 04.11.2021 14:00

Social Studies, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00

Health, 04.11.2021 14:00

Social Studies, 04.11.2021 14:00


Mathematics, 04.11.2021 14:00

English, 04.11.2021 14:00

Mathematics, 04.11.2021 14:00