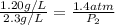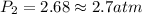Chemistry, 29.07.2019 13:00, ashleyann3052
To increase the solubility of a gas at constant temperature from 1.20 g/l, at 1.4 atm, to 2.3 g/l, the pressure would have to be increased to 2.7 atm 0.7 atm 0.37 atm 1.37 atm

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
Do you know the correct answer?
To increase the solubility of a gas at constant temperature from 1.20 g/l, at 1.4 atm, to 2.3 g/l, t...
Questions in other subjects:


Mathematics, 16.04.2021 21:00

Mathematics, 16.04.2021 21:00

Mathematics, 16.04.2021 21:00

English, 16.04.2021 21:00

Mathematics, 16.04.2021 21:00


History, 16.04.2021 21:00



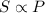

 = initial solubility of gas = 1.20 g/L
= initial solubility of gas = 1.20 g/L
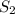 = final solubility of gas = 2.3 g/L
= final solubility of gas = 2.3 g/L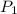 = initial pressure of gas = 1.4 am
= initial pressure of gas = 1.4 am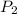 = final pressure of gas = ?
= final pressure of gas = ?