
Chemistry, 29.07.2019 13:30, aurelio1121
Radon (rn) is the heaviest and the only radioactive member of group 8a(18), the noble gases. it is a product of the disintegration of heavier radioactive nuclei found in minute concentrations in many common rocks used for building and construction. in recent years, health concerns about the cancers caused from inhaled residential radon have grown. if 1.00 × 1015 atoms of radium (ra) produce an average of 1.373 × 104 atoms of rn per second, how many liters of rn, measured at stp, are produced per day by 9.64 g of ra?

Answers: 1
Similar questions

Physics, 18.07.2019 07:50, sslider
Answers: 1

Chemistry, 10.10.2019 18:00, hdamelis30
Answers: 2

Physics, 25.11.2019 23:31, heavenwagner
Answers: 3
Do you know the correct answer?
Radon (rn) is the heaviest and the only radioactive member of group 8a(18), the noble gases. it is a...
Questions in other subjects:



Mathematics, 10.12.2020 01:10






Mathematics, 10.12.2020 01:10


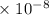 L.
L.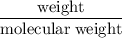
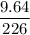
 Avagadro number
Avagadro number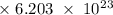
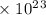
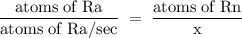
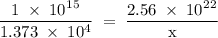
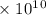 atoms/sec.
atoms/sec.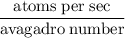
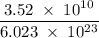 moles
moles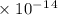 mol/sec.
mol/sec.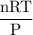
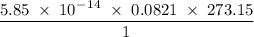
 liter/sec.
liter/sec.




