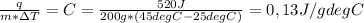Chemistry, 29.07.2019 14:00, 1tallison1
Will mark as brainliest. two hundred grams of a substance requires 0.52 kj of heat to raise its temperature from 25°c to 45°c. use the table to identify the substance. q = mc▲t. mass (m) is in grams. temperature is in degrees celsius. substance: specific heat (c) in joules per gram/degrees centigrade: water (ice) 2.05 iron 0.46 aluminium 0.90 gold 0.13 copper 0.39 ammonia (liquid) 4.70 ethanol 2.44 gasoline 2.22 water (liquid) 4.18 water (vapor) 2.08 air (25 degrees celsius) 1.01 oxygen 0.92 hydrogen 14.30 question options: water gasoline ammonia gold

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, breannaasmith1122
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
Do you know the correct answer?
Will mark as brainliest. two hundred grams of a substance requires 0.52 kj of heat to raise its temp...
Questions in other subjects:




Mathematics, 28.01.2020 13:39


Mathematics, 28.01.2020 13:40




Mathematics, 28.01.2020 13:40