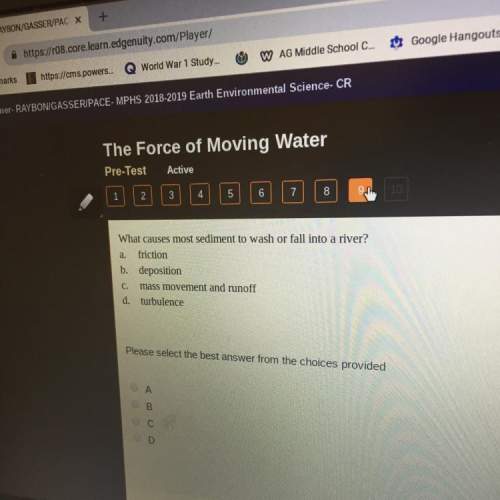
Chemistry, 29.07.2019 16:30, littlepotato10
Use ksp=4.87×10−17 to calculate the solubility of iron (ii) hydroxide in pure water in grams per 100.0 ml of solution. g

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
Do you know the correct answer?
Use ksp=4.87×10−17 to calculate the solubility of iron (ii) hydroxide in pure water in grams per 100...
Questions in other subjects:


Mathematics, 22.04.2020 03:28

Biology, 22.04.2020 03:28


Health, 22.04.2020 03:28

Mathematics, 22.04.2020 03:28

Mathematics, 22.04.2020 03:28