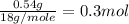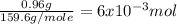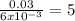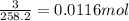Chemistry, 30.07.2019 02:00, maysahdabest
Suppose you begin with 1.50~g of the hydrate copper(ii)sulfate · x-hydrate (cuso4· x h2o), where x is an integer. after dehydration you find that you are left with 0.96~g of the an-hydrate cuso4. what is the unknown integer x. round your answer to the nearest integer, enter only an integer. suppose you begin with of the hydrate kal(so4)2 · 12h2o. after dehydration you find that you are left with 3.0~g of the an-hydrate kal(so4)2. how many grams did you start with? write the value to the correct number of significance figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, notkeandre9
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1



Chemistry, 23.06.2019 03:30, elijahjacksonrp6z2o7
In general metals get as you move from left to right across the periodic table.
Answers: 1
Do you know the correct answer?
Suppose you begin with 1.50~g of the hydrate copper(ii)sulfate · x-hydrate (cuso4· x h2o), where x i...
Questions in other subjects:








Mathematics, 25.06.2019 15:50

Mathematics, 25.06.2019 15:50