
Chemistry, 30.07.2019 02:00, razielcornils04
This reaction was monitored as a function of time: ab --> a + b a plot of 1/[ab] versus time yields a straight line with slope of -0.055/m*s. a) what is the value of the rate constant (k) for this reaction t this temperature? b) write the rate law for the reaction. c) what is the half-life when the initial concentration is 0.55m? d) if the initial concentration of ab is 0.250m, and the reaction mixture initially contains no products, what are the concentrations of a and b after 75s?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 07:30, tntaylor862
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
Do you know the correct answer?
This reaction was monitored as a function of time: ab --> a + b a plot of 1/[ab] versus time yi...
Questions in other subjects:


Mathematics, 13.04.2021 15:50





Mathematics, 13.04.2021 15:50

English, 13.04.2021 15:50


English, 13.04.2021 15:50


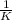 .
. 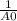
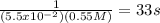
![\frac{1}{t} [ \frac{1}{[A]} - \frac{1}{[Ao]} ]](/tpl/images/0148/8063/927e3.png)




