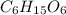Chemistry, 30.07.2019 02:00, sunshinekisses
Determine the molecular formula of a compound that has a molar mass of 183.2 g/mol and an empirical formula of c2h5o2.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 08:00, colbygreen6189
Identify the decay mode particle emitted from the th 234
Answers: 1


Chemistry, 23.06.2019 10:30, mikemofun9079
Silver is a white metal that is an excellent conductor. silver tarnishes when exposed to air and light. the density of silver is 10.49 g/cm3. the melting point is 962oc and the boiling point is 2000oc. a chemical property of silver is
Answers: 3
Do you know the correct answer?
Determine the molecular formula of a compound that has a molar mass of 183.2 g/mol and an empirical...
Questions in other subjects:

World Languages, 23.02.2021 14:00




Mathematics, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

English, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00