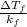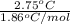Chemistry, 30.07.2019 02:00, vanessa051266
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 2.75 ∘c. what is the molal concentration of glucose in this solution? assume that the freezing point of pure water is 0.00 ∘c.

Answers: 1
Similar questions



Do you know the correct answer?
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 2.75 ∘c. what is the molal concentration...
Questions in other subjects:

Biology, 23.08.2019 02:00


Chemistry, 23.08.2019 02:00

English, 23.08.2019 02:00


History, 23.08.2019 02:00

Spanish, 23.08.2019 02:00



English, 23.08.2019 02:00


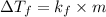
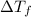 = difference in temperature =
= difference in temperature = ![[0 - (-2.75)]^{o}C](/tpl/images/0148/8342/d4fc3.png) =
=