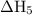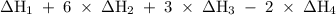Chemistry, 30.07.2019 13:00, emacwhaleng
Consider these reactions, where m represents a generic metal. 2m(s)+6hcl(aq)⟶2mcl3(aq)+3h2(g)δh1= −556.0 kj hcl(g)⟶hcl(aq) δh2=−74.8 kj h2(g)+cl2(g)⟶2hcl(g) δh3=−1845.0 kj mcl3(s)⟶mcl3(aq) δh4=−342.0 kj use the given information to determine the enthalpy of the reaction 2m(s)+3cl2(g)⟶2mcl3(s)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Do you know the correct answer?
Consider these reactions, where m represents a generic metal. 2m(s)+6hcl(aq)⟶2mcl3(aq)+3h2(g)δh1= −5...
Questions in other subjects:

English, 24.01.2021 20:10

History, 24.01.2021 20:10









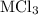 will be -5849 kJ.
will be -5849 kJ.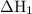 = -556 kJ
= -556 kJ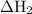 = -74.8 kJ
= -74.8 kJ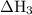 = -1845 kJ
= -1845 kJ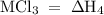 = -342.0 kJ
= -342.0 kJ