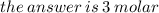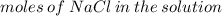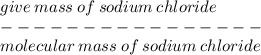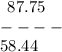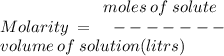Chemistry, 30.07.2019 16:30, kayla114035
Given that the molar mass of nacl is 58.44 g/mol, what is the molarity of a solution that contains 87.75 g of nacl in 500. ml of solution? use mc006-1.jpg. 0.333 m 0.751 m 1.50 m 3.00 m

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 10:00, alejandra216
You dissolve 8.65 grams of lead(l) nitrate in water and then you add 2 50 grams of aluminum. this reaction occurs 2ai(s)+ 3pb(no3)2(aq) -3pb(s)+ 2aino3la(aq) the theoretical yield of solid lead?
Answers: 1
Do you know the correct answer?
Given that the molar mass of nacl is 58.44 g/mol, what is the molarity of a solution that contains 8...
Questions in other subjects:

Mathematics, 26.02.2021 22:10





Mathematics, 26.02.2021 22:10



Mathematics, 26.02.2021 22:10

Mathematics, 26.02.2021 22:10