
Chemistry, 31.07.2019 06:30, smusisca53
Calculate the vapor pressure of a solution of 0.99 mol of cholesterol in 5.4 mol of toluene at 32°c. pure toluene has a vapor pressure of 41 torr at 32°c. (assume ideal behavior.)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
Do you know the correct answer?
Calculate the vapor pressure of a solution of 0.99 mol of cholesterol in 5.4 mol of toluene at 32°c....
Questions in other subjects:


History, 29.03.2020 21:07





Mathematics, 29.03.2020 21:07

Mathematics, 29.03.2020 21:07

Mathematics, 29.03.2020 21:07

Mathematics, 29.03.2020 21:07


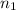 = 0.99 mol
= 0.99 mol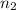 = 5.4 mol
= 5.4 mol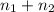
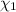 ) is as follows.
) is as follows.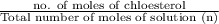
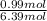
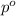 ) = 41 torr
) = 41 torr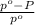
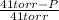 = 0.154
= 0.154 



