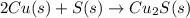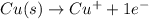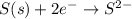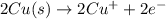Chemistry, 31.07.2019 06:30, Manuelperez1373
In the balanced redox reaction: 2 cu(s) + s(s) ® cu2s(s), how many electrons are gained or lost by each copper atom? select one: a. each copper atom gains two (2) electrons. b. each copper atom gains one (1) electron. c. each copper atom loses one (1) electron. d. each copper atom loses two (2) electrons.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 15:30, abdullaketbi71
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
Do you know the correct answer?
In the balanced redox reaction: 2 cu(s) + s(s) ® cu2s(s), how many electrons are gained or lost by...
Questions in other subjects:

Mathematics, 18.05.2021 20:10

Mathematics, 18.05.2021 20:10

Mathematics, 18.05.2021 20:10

Mathematics, 18.05.2021 20:10



History, 18.05.2021 20:10