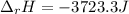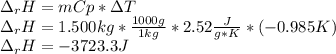Chemistry, 31.07.2019 12:00, anabelleacunamu
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbon dioxide (co2), and water (h2o). a chemist carries out this reaction in a bomb calorimeter. the reaction causes the temperature of a bomb calorimeter to decrease by 0.985 k. the calorimeter has a mass of 1.500 kg and a specific heat of 2.52 j/g•k. what is the heat of reaction for this system?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 23:40, tilievaughn14
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
Do you know the correct answer?
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbo...
Questions in other subjects:

Mathematics, 25.11.2019 04:31


Geography, 25.11.2019 04:31


Mathematics, 25.11.2019 04:31



Health, 25.11.2019 04:31

Social Studies, 25.11.2019 04:31

Mathematics, 25.11.2019 04:31