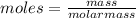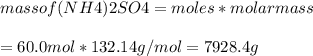Chemistry, 31.07.2019 20:00, chamyaparker
The chemical equation shows how ammonia reacts with sulfuric acid to produce ammonium sulfate. 2nh3(aq) + h2so4(aq) (nh4)2so4(aq) how many grams of ammonium sulfate can be produced if 60.0 mol of sulfuric acid react with an excess of ammonia? 1,020 g 3,970 g 5,890 g 7,930 g

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2
Do you know the correct answer?
The chemical equation shows how ammonia reacts with sulfuric acid to produce ammonium sulfate. 2nh3(...
Questions in other subjects:


Mathematics, 21.09.2019 00:30



Biology, 21.09.2019 00:30

English, 21.09.2019 00:30


Mathematics, 21.09.2019 00:30

Social Studies, 21.09.2019 00:30

Biology, 21.09.2019 00:30