
Chemistry, 01.08.2019 04:30, natalie407888
Chemistry ! : neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic mass of 40.0 g/mol. how would the number of atoms in a 1.0 mol sample of neon compare to the number of atoms in a 4.0 mol sample of argon? (2 points) a. the argon sample would have the same number of atoms as the neon sample. b. the argon sample would have twice as many atoms as the neon sample. c. the argon sample would have four times as many atoms as the neon sample. d. the argon sample would have eight times as many atoms as the neon sample.

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 12:30, pup88
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
Do you know the correct answer?
Chemistry ! : neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic m...
Questions in other subjects:


English, 19.01.2021 21:40

Mathematics, 19.01.2021 21:40

Mathematics, 19.01.2021 21:40


Mathematics, 19.01.2021 21:40



Business, 19.01.2021 21:40

Mathematics, 19.01.2021 21:40


 of particles.
of particles. contains=
contains=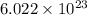 atoms
atoms  contains=
contains= atoms
atoms



