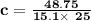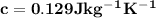Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1


Chemistry, 23.06.2019 01:00, carson9373
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
Do you know the correct answer?
As a 15.1-gram sample of a metal absorbs 48.75 j of heat, its temperature increases 25.0 k. what is...
Questions in other subjects:






Mathematics, 02.11.2019 03:31





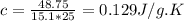
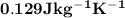 .
.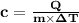
 is the change in temperature = 25 Kelvin
is the change in temperature = 25 Kelvin