Chemistry, 01.08.2019 12:00, mcclendoncassandra
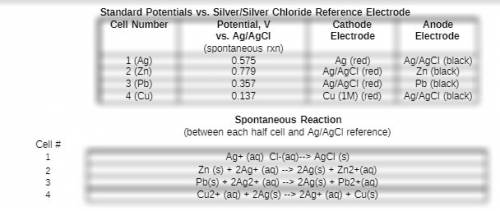
Based on your measured potential for this cell and the literature value for the standard reduction potential for the ag/agcl reference electrode, what would you expect the overall potential to be for the spontaneous reaction between your cu2+/cu electrode and a standard hydrogen eletrode? type your calculation for the expected standard reduction potential vs the she as well as the % error between this value and the literature value.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, muncyemily
If a 60-g object has a volume of 30 cm3, what is its density? 2 g/cm3 0.5 cm3/g 1800 g * cm3 none of the above
Answers: 3


Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1
Do you know the correct answer?
Based on your measured potential for this cell and the literature value for the standard reduction p...
Questions in other subjects:

Mathematics, 08.07.2019 15:30

Mathematics, 08.07.2019 15:30


Health, 08.07.2019 15:30


Spanish, 08.07.2019 15:30

Mathematics, 08.07.2019 15:30



History, 08.07.2019 15:30