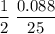Chemistry, 01.08.2019 16:00, cia196785920
Consider the reaction 2 hbr (g) à h2 (g) + br2 (g) a. express the rate of the reaction in terms of the change in concentration of each of the reactants and products. b. in the first 25.0 s of this reaction, the concentration of hbr drops from 0.600 m to 0.512 m. calculate the average rate of the reaction during this time interval.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1
Do you know the correct answer?
Consider the reaction 2 hbr (g) à h2 (g) + br2 (g) a. express the rate of the reaction in terms of t...
Questions in other subjects:



Physics, 11.10.2021 14:00

Mathematics, 11.10.2021 14:00

Mathematics, 11.10.2021 14:00




Mathematics, 11.10.2021 14:00


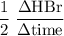 =
= 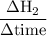 =
= 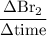 .
. 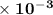 M/s.
M/s.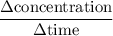
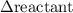 has been the change in reactants concentration, and
has been the change in reactants concentration, and 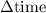 has been the change in the time.
has been the change in the time. = 0.60 M - 0.512 M
= 0.60 M - 0.512 M