
Chemistry, 02.08.2019 06:00, RickyGotFanz4867
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium constant, kc. if, at this temperature, 2.20 mol of a and 3.70 mol of b are placed in a 1.00-l container, what are the concentrations of a, b, and c at equilibrium?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, aeverettpdzrvo
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 21.06.2019 17:10, ladypink94
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 22.06.2019 00:00, 2024daisjavien
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
Do you know the correct answer?
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium consta...
Questions in other subjects:

Social Studies, 15.11.2021 22:10

Mathematics, 15.11.2021 22:10


English, 15.11.2021 22:30

Mathematics, 15.11.2021 22:50

English, 15.11.2021 23:00


Mathematics, 15.11.2021 23:10

Mathematics, 15.11.2021 23:20

History, 15.11.2021 23:20


![[C]_{eq}=4(0.733M)=2.932M](/tpl/images/0160/7486/db26c.png)
![[A]_{eq}=2.20M-3(0.733M)=0.001M](/tpl/images/0160/7486/5ae76.png)
![[B]_{eq}=3.70M-2(0.733M)=2.23M](/tpl/images/0160/7486/3f04f.png)
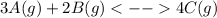
![[A]_0=\frac{2.20mol}{1.00L} =2.20M](/tpl/images/0160/7486/dc9d9.png)
![[B]_0=\frac{3.70mol}{1.00L} =3.70M](/tpl/images/0160/7486/d8b18.png)
![Kc=\frac{[C]_{eq}^4}{[A]_{eq}^3[B]_{eq}^2}](/tpl/images/0160/7486/3c943.png)
 , one obtains:
, one obtains: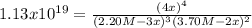
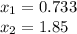
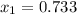 as the other one produces a negative concentration of A at equilibrium, therefore, the requested concentration turn out into:
as the other one produces a negative concentration of A at equilibrium, therefore, the requested concentration turn out into:



