
Chemistry, 02.08.2019 09:30, sammamamam7905
The atomic masses of 79 br 35 (50.69 percent) and 81 br 35 (49.31 percent) are 78.9183361 amu and 80.916289 amu, respectively. calculate the average atomic mass of bromine. the percentages in parentheses denote the relative abundances.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2


Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
Do you know the correct answer?
The atomic masses of 79 br 35 (50.69 percent) and 81 br 35 (49.31 percent) are 78.9183361 amu and 80...
Questions in other subjects:

History, 11.11.2020 01:30


Mathematics, 11.11.2020 01:30

Health, 11.11.2020 01:30


History, 11.11.2020 01:30


Mathematics, 11.11.2020 01:30


Biology, 11.11.2020 01:30


 . This yields an average atomic mass of 79.903 amu. This procedure is general; whenever we have many types F of a specific genre G, the mean of the genre G can be calculated by:
. This yields an average atomic mass of 79.903 amu. This procedure is general; whenever we have many types F of a specific genre G, the mean of the genre G can be calculated by: 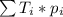
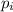 is the proportion of type i in the genre G. In this example, Br79 and Br81 are the 2 types and the genre is Bromine.
is the proportion of type i in the genre G. In this example, Br79 and Br81 are the 2 types and the genre is Bromine.



